Wet Chemistry

Who hasn't experimented with growing beautiful blue copper-sulphate crystals from solution as a child? That material is actually a very interesting quantum spin chain system. In fact, many quantum magnetic materials are grown from solution [see
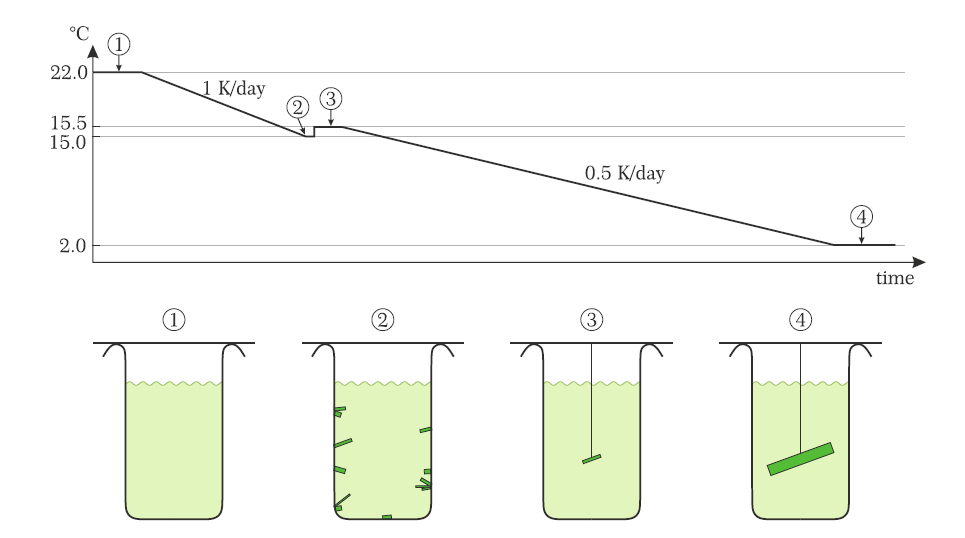
external page T. Yankova,D. Hüvonen,S. Mühlbauer,D. Schmidiger,E. Wulf,S. Zhao,A. Zheludev ,T. Hong,V.O. Garlea,R. Custelcean &G. Ehlers, Phylosophical Magazine 92, 2629 (2012)]. The process is in theory not complicated. In practice though, there is a lot of trial and error involved in selecting the optimal growth parameters. One is rewarded with beautiful samples, some of which are displayed in our quantum materials Gallery. The picture above show thermal-gradient growth of two other quantum spin chain materials, namely K2CuSO4Cl2and K2CuSO4Br2, as described in detail in the PhD tthesis of Dr. manuel Hälg (2015).

Many of the quantum magnetic materials grown with wet-chemistry methods, like the dimer system (C5H6N2F)2CuCl4 grown with the slow-cooling technique [external page D. Blosser, PhD thesis (2019)], contain hydrogen. This is a huge problem for neutron scattering, because protons have a huge incoherent cross section. Neutrons are scattered out of the sample and contribute to huge noise levels. The only way around is to use deuterium-enriched samples. Deuterated versions of some organic ligands can be procurred commercially. For others, like in the case of (C5H6N2F)2CuCl4 we perform catalytric deuteration in specialized "pressure cookers" in our lab. Samples grown from deuterated starting chemicals are mounted for neutron scattering experiments.